Trulance established safety and tolerability across two indications1
Studied in more than 3100 patients across 4 double-blind, placebo-controlled, 12-week studies.
Treatment-emergent diarrhea was observed in ≤5% of patients
No additional adverse events (AEs) occurred at an incidence of ≥2% in patients treated with Trulance and at an incidence greater than placebo1
Most common AEs in IBS-C1
IBS-CAE
(n=723)
(n=726)
Diarrhea
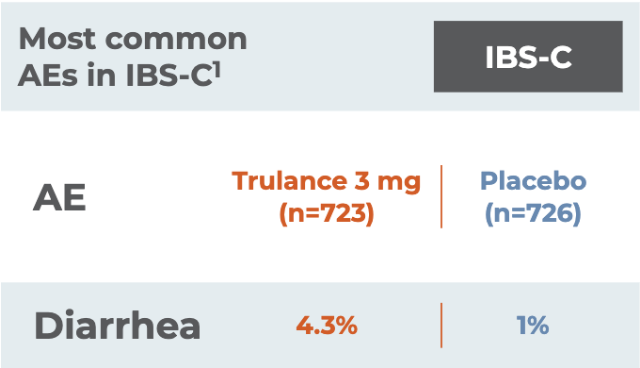
Severe diarrhea was reported in 1% of patients receiving Trulance vs 0.1% of placebo-treated patients
2.5% discontinuation rate for Trulance vs 0.4% for placebo due to AEs
1.2% discontinuation rate due to diarrhea vs 0% for placebo
Reported in at least 2% of Trulance-treated patients with IBS-C and at an incidence greater than placebo1
Most common AEs in CIC1
CICAE
(n=863)
(n=870)
Diarrhea
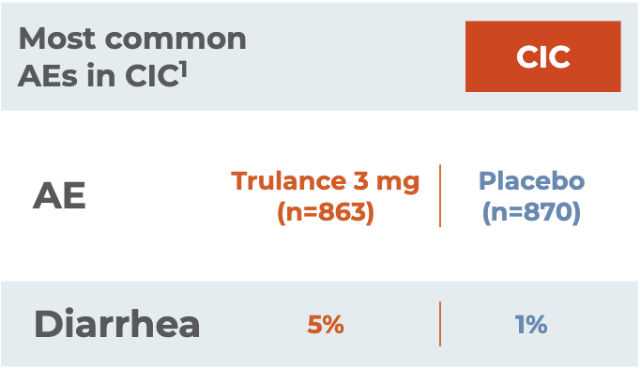
Severe diarrhea was reported in 0.6% of patients receiving Trulance vs 0.3% of placebo-treated patients
4% discontinuation rate for Trulance vs 2% for placebo due to AEs
2% discontinuation rate due to diarrhea vs 0.5% for placebo
Reported in at least 2% of Trulance-treated patients with CIC and at an incidence greater than placebo1

If severe diarrhea occurs, suspend dosing and rehydrate the patient1

No drug-drug interactions observed1

Minimally absorbed with negligible systemic availability1
Indication
Trulance (plecanatide) 3 mg tablets is indicated in adults for the treatment of Chronic Idiopathic Constipation (CIC) and Irritable Bowel Syndrome with Constipation (IBS-C).
IMPORTANT SAFETY INFORMATION
WARNING: RISK OF SERIOUS DEHYDRATION IN PEDIATRIC PATIENTS
Trulance® is contraindicated in patients less than 6 years of age; in nonclinical studies in young juvenile mice, administration of a single oral dose of plecanatide caused deaths due to dehydration. Avoid use of TRULANCE in patients 6 years to less than 18 years of age. The safety and effectiveness of TRULANCE have not been established in patients less than 18 years of age.
TRU.0042.USA.24
